My Order
Your cart is empty.
Have an account?
Log in here.

Our doctors and nursing teams are equipped to support you, your family, and your business with quality care. Connect in minutes with board-certified doctors over live audio/video and have your questions answered. Create a treatment plan with our network of physicians and have true 24/7 concierge care.
COVID-19 Rapid Testing in select regions with BD Veritor and Abbott Binax Now Antigen testing:
Schedule Rapid COVID-19 Testing
COVID-19 Rapid Molecular (PCR) Same-Day COVID19 Testing in select regions:
Nurse Administered with results in as little as 15 minutes. Results interpreted by a physician.
AVAILABLE IN SELECT CITIES ONLY
Quick Facts on RAPID COVID19 Testing:
The rapid COVID test is administered at the point of care by a gentle anterior nasal swab by our registered nursing team in the comfort and privacy of your home. The rapid antigen test can return near-immediate results showing whether the patient is infected with acute COVID-19 the same day (in as little as 15 minutes), as opposed to waiting an hour (same day PCR in select cities) or 24-72hrs for a PCR test.
Schedule Rapid COVID-19 Testing
THE IV DOC team uses the BD VERITOR Plus System and Abbott Binax Now for the rapid COVID-19 Antigen test. This is a state-of-the-art technology to detect nucleocapsid proteins, indicating the presence of SARS-CoV-2. This quick, simple, and easy-to-use test provides objective results for the utmost possible accuracy.
The rapid COVID test reveals only whether the COVID-19 virus is currently present. It does not test whether or not the patient has previously been infected by COVID-19, or whether any other viruses are currently present. The test does not react to the presence of any other seasonal coronaviruses.
Besides the rapid covid test we also currently offers nasal swab PCR testing for COVID-19 (needed for some international travel), as well as antibody testing to determine whether you have been infected with the coronavirus in the past.
Rapid Antigen COVID-19 Testing
rt-PCR Test
COVID-19 Testing: Viral Levels vs. Onset of Symptoms

Most insurance carriers will provide you reimbursement.
Ask our billing team for a coded receipt following your service.
Please allow a minimum of 72hours to receive your coded receipt.
Nurse Administered with results in as little as 24-72 hours. Our team or Physicians will always be available to assist you interpreting your results.
Most insurance carriers will provide you reimbursement.
Ask our billing team for a coded receipt following your service.
Please allow a minimum of 72hours to receive your coded receipt.
Just like an in-person visit, the doctor takes your history and symptoms, performs an exam and may recommend treatment, which may include at home or at office COVID-19 Testing and even antibody testing performed by a Registered Nurse under the supervision of the treating physician.
Laboratory partnerships have been made for accurate and fast PCR results. Our team of physicians will provide you with test results in as little as 36hrs. The test is simple and will be completed at your home or office by a Registered Nurse, under the visual guidance of your treating physician. Reduce unnecessary exposure and ask your physician if COVID-19 research testing is right for you.
THE I.V. DOC is here to support your return to work with strategies to reduce risks of viral outbreak. Our goal is to provide a safe working environment for your employees. Screening for active infection and symptoms as well as contact tracing will play critical roles in reducing the chance of an office resurgence of cases. Employers accross the U.S. have entrusted THE I.V. DOC to provide employee screening following CDC and OSHA guidelines while respecting individual rights and privacy considerations associated with testing.
Please email us for business return to work pricing
COVID-19 (Molecular) rt-PCR TEST
FAQ: COVID-19 qPCR Test (Coronavirus - SARS-CoV-2)
What does the labs test identify?
The labs COVID-19 qPCR Test identifies the SARS CoV-2. This testing method utilizes rt-PCR (real-time reverse transcriptase PCR) technology, and will confirm the virus is present. Although, it is unclear the exact timelines of all stages.
Does the labs test measure viral antibodies or viral load?
The labs test is not an antibody test. The labs test confirms if the virus is currently present in the sample and does NOT measure antibody levels or viral load.
What is the labs turnaround time (TAT) for results?
Once the sample collection kit is received, the turnaround time (TAT) for results is approximately 24-72 hours. Delays can occur so please plan accordingly.
Who receives the results?
The clinician assigned by THE I.V. DOC will receive an email with a secure link to their Clinician Portal. The clinician will then login to their secure Clinician Portal to generate the report. An SMS or Email will be sent to you prompting you to login to review your results. Doctors are available 24/7 to answer any of your questions.
How do you take the test?
Nasopharyngeal, oropharyngeal, saliva, and anterior nare swabs are all available. Detailed collection instructions will be provided by your nurse and physician.
In clinical lab terms, what is the collection procedure?
The COVID-19 virus nucleic acid will be extracted from throat swab samples, 1-step reverse transcription of RNA from the virus and amplification via PCR for a combined method known as real-time reverse transcriptase PCR (RT-PCR). An internal positive control will be included to ensure that the test performed correctly and that the RT-PCR reaction has not been inhibited.

Doctors are equipped to support you and your family with quality care. Connect in minutes with board-certified doctors over live video and have your questions answered. Create a treatment plan with a doctor and get better faster!
Just like an in-person visit, the doctor takes your history and symptoms, performs an exam and may recommend treatment, which may include at home COVID-19 Testing and even antibody testing performed by a Registered Nurse under the supervision of the treating physician.
Our partner laboratories are certified under CLIA to perform waived, high-complexity testing in addition there methods are listed as laboratories that have notified the FDA that they have validated COVID-19 diagnostic tests. It's important to know that a negative test result do not preclude acute SARS-CoV-2 infection. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
Results and treatment time may vary. Medication and third party laboratory testing may be provided, only after a consultation by a licensed medical provider.
Antibody Testing - BLOOD TEST
What are IgM and IgG antibodies?
IgM is usually the first, specific antibody type generated by the body in response to infection. Your body usually generates these antibodies as its first line of defense against a foreign virus and can develop as soon as 3-5 days.
IgG antibody “is a protein that the body produces in the late stages of infection (possibly 21 days or greater) and may remain for up to months and possibly years after a person has recovered.”
IgM and IgG fight infections by targeting specific antigens on the surface of the SARS-nCoV-2 virus.
How is the Antibodies (Blood) test different from the Rt-qPCR,DNA (Swab) test?
The RT-qPCR,DNA test determines if the individual is infected with SARS-CoV-2 and considered to be able to transmit the disease (a positive test) or is negative for the virus. This test cannot tell whether a person is immune from past infection or has yet to be exposed and is still in danger.
The IgG & IgM antibodies test is based on the quantitative detection of IgM and IgG that are specifically generated by the body in response to SARS-CoV-2 infection. This can be used to determine recent & past exposure. But should not be used as a means of determining active infection alone.
Researchers said having the IgG antibodies gives you protection from the virus -- but it doesn’t mean you’re immune for the rest of your life. You could get sick again. Still, they’re calling the test a milestone towards getting life back to normal.
Clinical Significance of Utilizing the DNA (swab) test along with the Antibodies (blood) test.
Antibody tests for COVID-19 cannot confirm the presence of the virus in your system at the present day. It can only tell whether you have been exposed in the past or if you have never been exposed to SARS-CoV-2. Consequently, it should only be used alone as a screening test and should be used in tandem with a genetic-based test to determine a complete status.
Clinical Significance (DNA + Antibodies)
|
COVID-19 Real Time TR-qPCR(swab) |
IgM Antibody (blood) |
IgG Antibody (blood) |
Clinical Significance |
|---|---|---|---|
|
Not Detected |
Not Detected |
Not Detected |
Patient was most likely not exposed to SAR-CoV-2 |
|
Detected |
Not Detected |
Not Detected |
Patient may be in window period of infection |
|
Detected |
Detected |
Not Detected |
Patient may be in early stage of infection |
|
Detected |
Not Detected |
Detected |
Patient may be in later or recurrent stage of infection |
|
Detected |
Detected |
Detected |
Patient is in active stage of infection |
|
Not Detected |
Detected |
Detected |
Patient may be in the recovery stage of infection |
|
Not Detected |
Detected |
Not Detected |
Patient may be in early stage of infection |
|
Not Detected |
Not Detected |
Detected |
Patient may have had past infection, recovered and possibly immune |
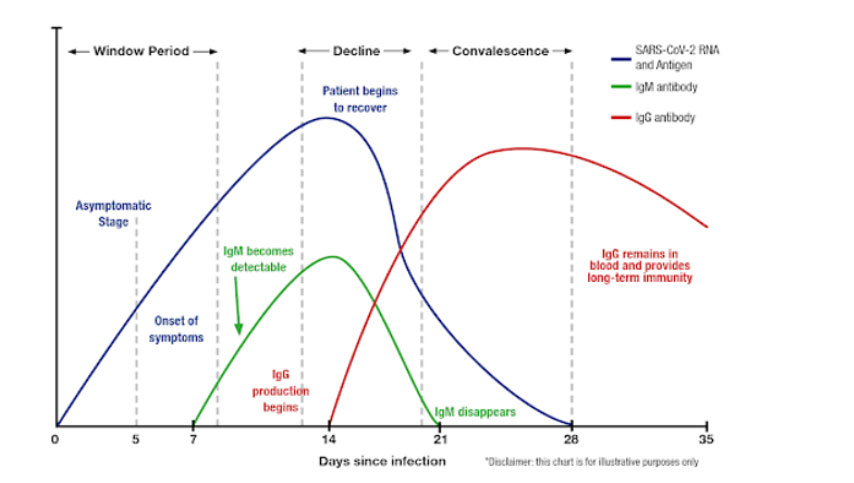
Variation of the Levels of SARS-CoV-2 RNA and Antigen, IgM and IgG after infection.
Our Lab Partners IgM & IgG Qualititave and possible Quantitative Antibody Tests
Our lab partners IgG & IgM testing is performed in their CLIA High Complexity specialty lab on dedicated, automated instrumentation which is listed with the FDA and testing is EUA authorized.
Our lab partners provide a qualitative report and many times a quantitative report, which not only informs of detection, but also quantifies the amount of antibodies detected.
The FDA has provided our lab partners Emergency Use Authorization (EUA) for COVID19 Tests. Our partner laboratories are certified under CLIA to perform high-complexity testing. CLIA waivers are obtained for Rapid Antigen Testing. It's important to know that a negative Rapid Antigen Test, PCR Test, or Antibody test result do not preclude acute COVID19 (SARS-CoV-2) infection. If an acute infection is suspected, direct testing for COVID-19 is may be necessary rapid antigen testing or molecular - PCR testing. Results from antibody testing should not be used to diagnose or exclude acute COVID-19 infection. Again, a negative result on any test provided does not exclude the possibility of COVID19 infection as no tests are perfect! Positive results may be due to past or present infection with non-COVID-19 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
Medication, testing, and third party laboratory testing may be provided, only after a consultation by a licensed medical provider.
(C)2020 The I.V. Doc. All Rights Reserved. Results and treatment time may vary. Medication and third party laboratory testing may be provided, only after a consultation by a licensed medical provider.
“You can’t afford to be sick in NYC… Thank you!!!”
- George, Dallas
“I feel like a new man Thank you I.V. Doc”
J.S. - Manhattan
“For those asking if The I.V. Docs services are worth it, the answer is a big fat YES! Thanks for bringing me back to life!”
- Kristina, PA
“Thanks... I'm feeling great!! You guys saved my vacation and my life. I can't thank you enough.”
- Stacy, Colorado
“At my office at 9, trading by 9:30…Saviors!”
- John, Brooklyn
“I feel like a new woman. Your staff was excellent thank you so much!”
- Tracy, San Francisco
“The best thing to happen to Chicago! (Go Cubs)”
- David, Chicago
“You saved my Vegas trip! When in Vegas always plan for The I.V. Doc!”
Sam, Las Vegas
“THANK YOU!!!!! I was able to go home for Father's Day without feeling ill AND I was able to stuff my face with filet mignon which would not have been possible without you guys!! Thanks again!”
- Kat, NYC
“The Flu Relief was literally life saving! Thanks IV DOC”
Heather, New York
“B12!! Weight loss, energy, focus! See you next week.”
- Rachel, NYC
“Got revived… and now I feel like I could run a marathon”
- Wes, Manhattan
Results may vary.

Call anytime to schedule by phone:
844-843-4836